
Benzoic Acid is a colourless crystalline solid and a simple carboxylic acid. The name is derived from gum benzoin, which was for a long time its only known source. Benzoic Acid occurs naturally in many plants and it serves as an intermediate in the biosynthesis of many secondary metabolites. Salts of Benzoic Acid are used as food preservatives and benzoic acid is an important precursor for the industrial synthesis of many other organic substances. The salts and esters of benzoic acid are known as benzoates. - Wikipedia
Although benzoic acid has many uses outside of bomb calorimetry, we will touch on the subject of its use in calorimetry. Benzoic acid is relatively non-toxic and occurs naturally in plants.
Appreciable amounts of it has been found in most berries (around 0.05%) and is formed in apples after infection with a specific fungus.
LABORATORY USE
Due to its constant value, it is the ideal substance for calibration in bomb calorimeters. For this purpose, we press the powdery substance into tablets of approximately 0.5 grams and 1.0 grams, with a calorific value of approximately 26.454KJ/g.
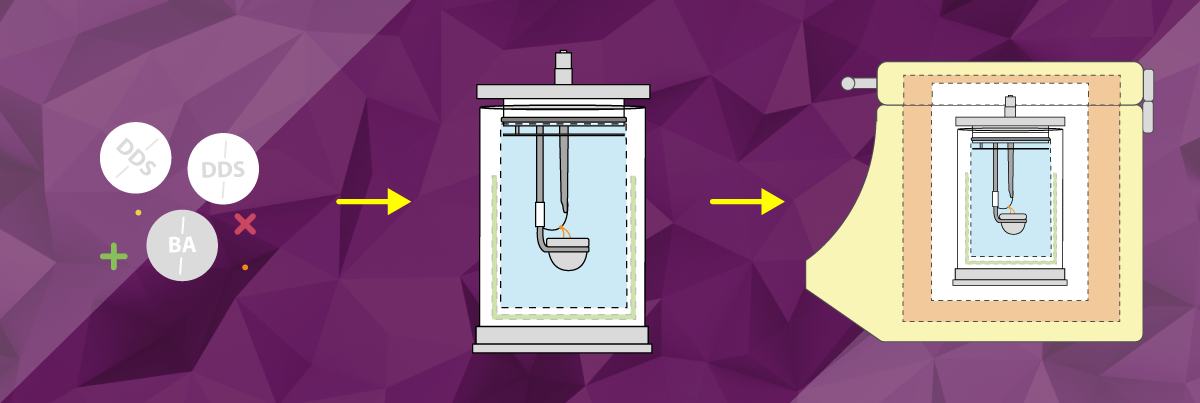
CALIBRATION OF OXYGEN BOMB CALORIMETERS
The calibration of an oxygen bomb calorimeter system (also called standardization) is done using a tablet of benzoic acid. DDS Calorimeters manufacture our own benzoic acid tablets which are suitable for use in all our oxygen bomb calorimeter systems. During this calibration process, a known amount of heat is introduced into the calorimeter by the combustion of a benzoic acid tablet. This produces the energy equivalent of the calorimeter for a specific temperature rise.
Before an unknown sample can be tested, the heat capacity or energy equivalent (EE or E) of the bomb calorimeter must be determined. The value resulting from the burning of the calibration standard Benzoic Acid represents the heat capacity of the calorimeter (including the bomb vessel, insulating polystyrene or ATC and all other parts of the calorimeter system). Energy equivalents are determined at regular intervals by burning a sample of the standardization material with a known heat of combustion (Benzoic Acid at 26.454KJ) under controlled and reproducible operating conditions.

DESIRABLE PROPERTIES FOR BENZOIC ACID
Benzoic Acid tablets used for combustion calorimetry should adhere to the following requirements:
- It must be readily available in pure form
- It must be stable
- It must not be hydroscopic
- It must not be volatile
- It must be easily brought into a form suitable for combustion
- It should offer no unusual difficulties during combustion in the bomb
- The value of the heat of combustion is characterized with suitable accuracy
While DDS Calorimeters Benzoic Acid Tablets adhere to the above requirements, it is also used for the following applications:
- It is advantageous for tests involving materials that are difficult to handle or ignite and assists with the burning of the sample during a determination.
BENZOIC ACID CHARACTERISTICS
Benzoic acid is soluble in water, alcohol, benzene, and ether. Its aqua solution is weakly acidic. It is non-toxic and stable under ordinary conditions. Benzoic acid occurs naturally in many plants and resins. It is also detected in animals.
APPEARANCE OF BENZOIC ACID
Benzoic acid manufactured and supplied by DDS Calorimeters is supplied in a colourless to white solid tablet/pellet with aromatic-like odour. Soluble in alcohol, ether, acetone, benzene, chloroform. Partially soluble in water.
DDS Calorimeters Benzoic Acid Tablets are supplied in the following sizes and quantities:
- 100 Tablets of 0.1g weight Benzoic Acid Tablets in glass bottle
- 150 Tablets of 0.5g weight Benzoic Acid Tablets in glass bottle

BENZOIC ACID SAFETY & PRECAUTIONS
- SWALLOWED - Accidental ingestion of material may be harmful; ingestion of more than 150g may be fatal or may produce serious damage to the health of the individual. Hazardous in case of ingestion.
- EYE CONTACT - There is evidence that material may produce eye irritation in some people and produce eye damage 24 hours or more after instillation. Severe inflammation may be expected with pain. Hazardous in case of eye contact (irritant).
- SKIN CONTACT - The material may cause moderate inflammation of the skin either following direct contact or after a delay of some time. Open cuts, abraded or irritated skin should not be exposed to this material. Examine the skin prior to the use of the materials and ensure that any external damage is suitable protected. Hazardous in case of skin contact (irritant).
- INHALED - Benzoic acid in large doses is toxic to lungs, the nervous system, and mucous membranes. Repeated or prolonged exposure to Benzoic Acid can produce target organ damage.
BENZOIC ACID STORAGE AND HANDLING
Workers in immediate area of machinery should wear eye and skin protection (i.e. safety glasses, dust respirator).
Avoid personal contact (use the stainless steel tweezers provided with the installation kit to handle Benzoic Acid Tablets - this reduces the risk of getting moisture from skin contact onto the tablet).
Wear protective clothing when working with volatile combustible samples.
Do not ingest Benzoic Acid Tablets (although it is used as a preservative and occurs naturally in plants, for the safety of the operator do not ingest tablets. Used for bomb calorimetry purposes only.)
Do not breathe in dust (DDS Calorimeters tablets are already compressed into the table form for easy handling and use in our bomb calorimeter systems).
Avoid contact with skin and eyes as irritation may occur with sensitive skin.
Store Benzoic Acid in a plastic or glass container (DDS Calorimeters Benzoic Acid Tablets are already packaged in plastic/glass containers. Ensure lid is screwed on tightly and sealed when storing). Check that all containers are clearly labelled for safety purposes.
The estimated shelf/storage life of Benzoic Acid Tablets is approximately 5 years. Store away from incompatible substances. Store at room temperature (15/25°C recommended). Keep closed and protected from direct sunlight and moisture. Keep away from heat and sources of ignition. Keep container tightly closed, in a cool, well-ventilated area.
DISPOSAL CONSIDERATIONS
- All waste must be handled in accordance with local, state and federal regulations.
- Shelf life considerations should also be applied.
- Note that properties of a material may change in use and recycling/reuse may not always be appropriate.
- Recycle wherever possible
- Sweep up, then place into a suitable container for disposal.
- Avoid generating dusty conditions.
CONCLUSION
In simple terms: The mass of a Benzoic Acid Tablet is measured and then ignited in a calorimeter. Then all further unknown samples are compared to the calibration. Of course, if any part of the calorimeter is changed then the calibration must be repeated. Clean out crucibles after every firing and replace with a new Benzoic Acid Tablet before proceeding with the next firing during a calibration.
The calibration is repeated once a day or every week to make sure nothing in the method or apparatus is changed.

BENZOIC ACID TABLETS
DDS Calorimeters manufacture and supply our own range of Benzoic Acid Tablets for use in the CAL2K and CAL3K Oxygen Bomb Calorimeter Systems.
To purchase any of the options listed above contact your nearest agent or email us at calo@ddystems.co.za for more information or to request a free quote.
CONTACT AN AGENT


Leco Bomb Calorimeter NEEDED
Assy Vessel Combustion II 1 EA
Acid Benzoic LCRM Tablet 2 BT
Wire Igniter Fuse Thread (5/pk) 5 PAC
Fuse Thread Cotton (375/pk) 5 EA
Assy Stand Vessel Combustion 1 PAC
Hi there.
I have sent your enquiry to our sales manager to best assist you with your analytical needs.
Kind regards, Jess. – DDS Team